BENGALURU: An anti-cancer device developed by a Bengaluru-based medical engineer has got the honour of being called a ‘breakthrough device’ by the American Food and Drug Administration Department.
“We are pleased to inform you that your device and proposed indication for use meet the criteria and have been granted designation as a breakthrough device,” states a communique from the FDA wing to Shreis Scalene Sciences, the company that had taken the device to the US, reported Times of India.
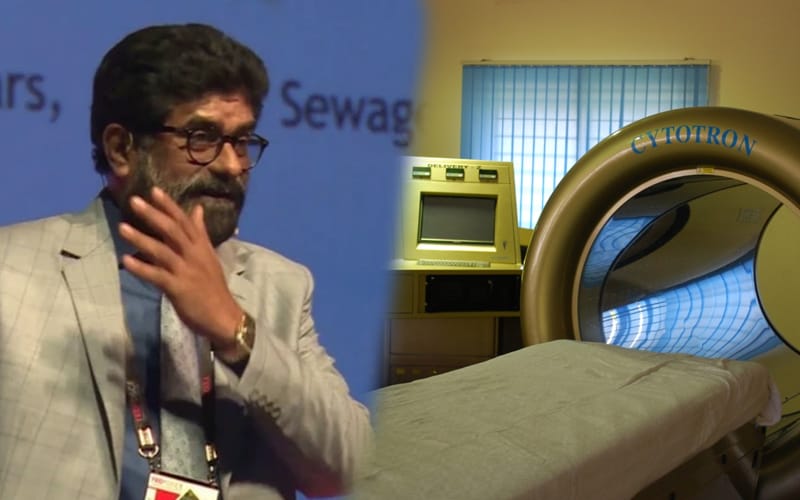
‘Cytotron’, a medical invention by Rajah Vijay Kumar has been given this tag as it helps in the treatment of liver, pancreatic and breast cancers.
Hope it offer a breakthrough to cancer patients
Kumar, who is the Chairman and Chief Scientific Officer at the Centre for Advanced Research and Development (CARD) feels great after receiving the ‘breakthrough device’ designation from the USFDA.
“It is a great feeling that after so many years of hard work, against all odds, an institution like the USFDA is designating our work as a breakthrough in the treatment of three types of cancers,” Kumar said and “we hope it will also offer a breakthrough in the lives of cancer patients”
How Cytotron works
Specifically designed ‘Cytotron’ by Kumar took more than 30 years of continuous research at the Centre for Advanced Research and Development in Bhopal
‘Cytotron’ looks much like an MRI scanner machine, but uses fast radio bursts (FRB) that “intended to stop the cancer tissue from multiplying, growing and spreading.”
Made in India
The FDA also commended the fact that the components used in the development of ‘Cytotron’ device is all made in India with hardly any parts imported.
“The devices will all be made in India, given that there are hardly any imported components. And our American partner will take the device to the US. Cytotron is already an approved medical device and is in use in the UAE, Mexico, Malaysia and Hong Kong, among others,” Kumar said.